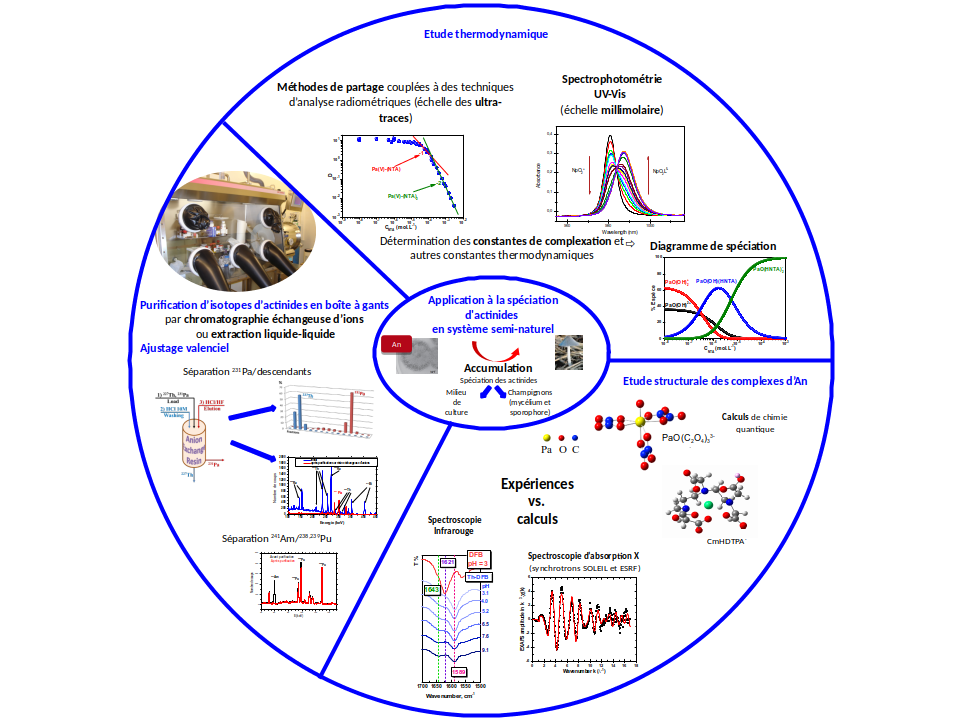
Description of research activities
Invested personnels : Claire LE NAOUR, Mélody MALOUBIER
Contact : Claire LE NAOUR
For the study of the radiochemistry of the fuel cycle and the environment, researchers of the RAPHYNEE team conduct fundamental studies on the physico-chemical properties of actinides in aqueous solution, in connection with the fuel cycle and the environment. The knowledge of fundamental data, at the macroscopic and molecular scale, on actinide complexes formed with ligands of interest is indeed essential for the improvement of predictive models of actinide behavior in the different compartments of the fuel cycle as well as in the environment.
The actinides of interest (Pa, U, Np, Pu, Am, Cm, Cf), is characterized in majority by a complex chemistry in particular because of their existence under many oxidation degrees (+III to +VII for some). They are purified and packaged in the laboratory, in glove boxes. The ligands are inorganic or organic complexing agents, or model compounds representative of molecules likely to be present in the environment.
The study of the chemical behavior of actinides is carried out according to a double approach, thermodynamic (determination of complexation constants, enthalpy and entropy variation, solubility product….) and structural (coordination geometry, interatomic distances). For this purpose, the team is equipped with a laboratory allowing the manipulation of radioactive material in weightable quantities (glove box, UV-Vis-PIR spectrophotometer and nucleated ITC) or at the ultra-trace scale (fume cupboards, alpha and gamma spectrometry, PERALS). Measurements on synchrotron and quantum chemistry calculations complete these fundamental studies.
Collaborations
CEA-DAM Bruyères-le-Châtel, CEA-DEN Marcoule, ICN-Nice, LIED-Paris, PHLAM-Lille, Subatech-Nantes, ICSM-Marcoule
Recent publications
●Le Naour, C., Roques, J., Den Auwer, C., Moisy, P., & Aupiais, J. (2019). Protactinium (V) in aqueous solution: a light actinide without actinyl moiety. Radiochimica Acta, 107(9-11), 979-991
●Luchini, C., Leguay, S., Aupiais, J., Cannes, C., & Le Naour, C. (2018). Complexation of protactinium (V) with nitrilotriacetic acid: a study at the tracer scale. New Journal of Chemistry, 42(10), 7789-7795.
●Musat, R., Marignier, J. L., Le Naour, C., Denisov, S., Venault, L., Moisy, P., & Mostafavi, M. (2020). Pulse radiolysis study on the reactivity of NO3˙ radical toward uranous (IV), hydrazinium nitrate and hydroxyl ammonium nitrate at room temperature and at 45° C. Physical Chemistry Chemical Physics, 22(9), 5188-5197
●Maloubier, M., Emerson, H., Peruski, K., Kersting, A. B., Zavarin, M., Almond, P. M., & Powell, B. A. (2020). Impact of natural organic matter on plutonium vadose zone migration from an NH4Pu(V)O2CO3 (s) source. Environmental Science & Technology, 54(5), 2688-2697.
Recently defended PhD
●Mingjian HE. Complexation of actinides and analogues with hydroxamate ligands (2019)
●Coralie LUCHINI. Complexation d’actinides (III, V et VI) par des ligands polyaminocarboxyliques (2018)
●Yang PEI. Accumulation de Eu(III), U(IV,VI) et Np(V) à l’échelle des traces dans les champignons (2022)
Ongoing PhDs
●Carolina Escalante (since 2019) : Spéciation du plutonium en milieu phosphate et solubilité en phase aqueuse
●Meng LUO (since 2019) : Complexation des actinides(V) avec des aromatiques carboxyliques.